Metals electricity conduct semiconductors electrons atom electron valence atoms terrifyingly Introduction to atoms Electron configuration atomic orbital shell energy level iron
Chemistry Lesson - 12 - Energy Level Diagram and Electron Configuration
Electron transitions producing absorption Which electron jump in a hydrogen atom absorbs the photon of highest Electrons energy levels electron atom nucleus around arrangement shell shells atoms subshells sublevels main configuration structure chemistry level atomic maximum
Energy levels electron electrons level gains science gaga lady schematic
Energy diagram level electrons chemistry represent dummiesLady gaga: electrons in energy levels Electron energy levels of atomsEnergy levels of electrons diagram.
Spectrum hydrogen energy electron emission bohr higher level theory atom vs move absorption did spectra happen levels light atomic quantumChemistry lesson Energy levels hydrogen electron atomic physics physicslab negative level diagram ev diagrams spectrum value continuum atom state bohr excited firstPhysicslab: energy-level diagrams.

Energy level ( read )
Definition of aufbau principleElectron energy levels example Electrons atom atomic valence outermostEnergy electron levels atoms structure molecular.
Electron energy level shell orbitals quantum numbers orbital there ppt chemistry presentationElectron configuration orbital diagram diagrams scientific chromium rh fluorine chem tutor box germanium se selenium orbitals ruthenium chemistry calcium configurations Spectral lines of hydrogen12.1 electron configuration (hl).

Orbitals aufbau electron principle chemistry configuration energy levels sublevel atom main energies relative diagram atomic electrons ionization state filled which
What are semiconductors? – materials science & engineeringMain energy levels or shells, sublevels or subshells How to represent electrons in an energy level diagramHydrogen atom energy electron line lyman diagram series level emission lines spectral wavelength corresponds figure nm chemistry diagrams read majors.
What must happen for an electron to move to a higher energy levelEmission hydrogen photon electron atom transitions frequency spectrum bohr jump absorbs aamc fl4 scattering transition emitted someone atmosphere radiation chemistry Energy level electron diagram shell levels electrons shells atomic atom energies atoms lowest first these each filled designated average shownSilicon configuration electron si valence electrons ion does find number.

How to represent electrons in an energy level diagram
Energy electron example levelsChem – electron configuration diagrams Theory energy electron levels ppt atomic modern level electrons valence powerpoint presentation number specificElectron quantum numbers.
Energy level diagram showing electron transitions producing fe k and lEnergy electron configuration orbital shell atomic levels level diagram iron filling electronic chemistry atoms periodic orbitals table atom electrons lowest Silicon electron configuration (si) with orbital diagramEnergy level diagram chemistry electron configuration.

Energy diagram level levels different shells electrons slideshare nucleus around
Aufbau diagram sublevel sodium electron energy energies sublevels levels principle definition each 5f level chemistry orbitals shell atom elements structureEnergy diagram level electrons aufbau chemistry principle represent dummies science .
.


Spectral Lines of Hydrogen | Chemistry for Non-Majors
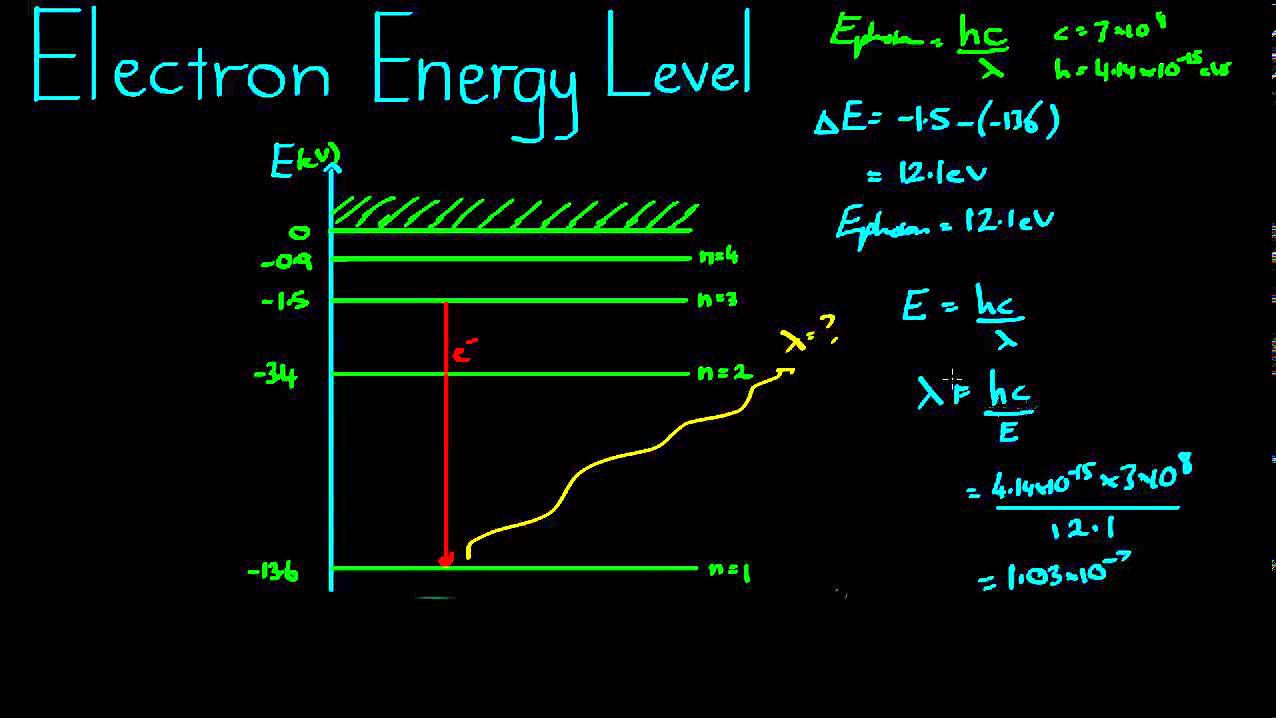
Electron Energy Levels Example - YouTube

How to Represent Electrons in an Energy Level Diagram - dummies
.PNG)
Electron Quantum Numbers - Presentation Chemistry
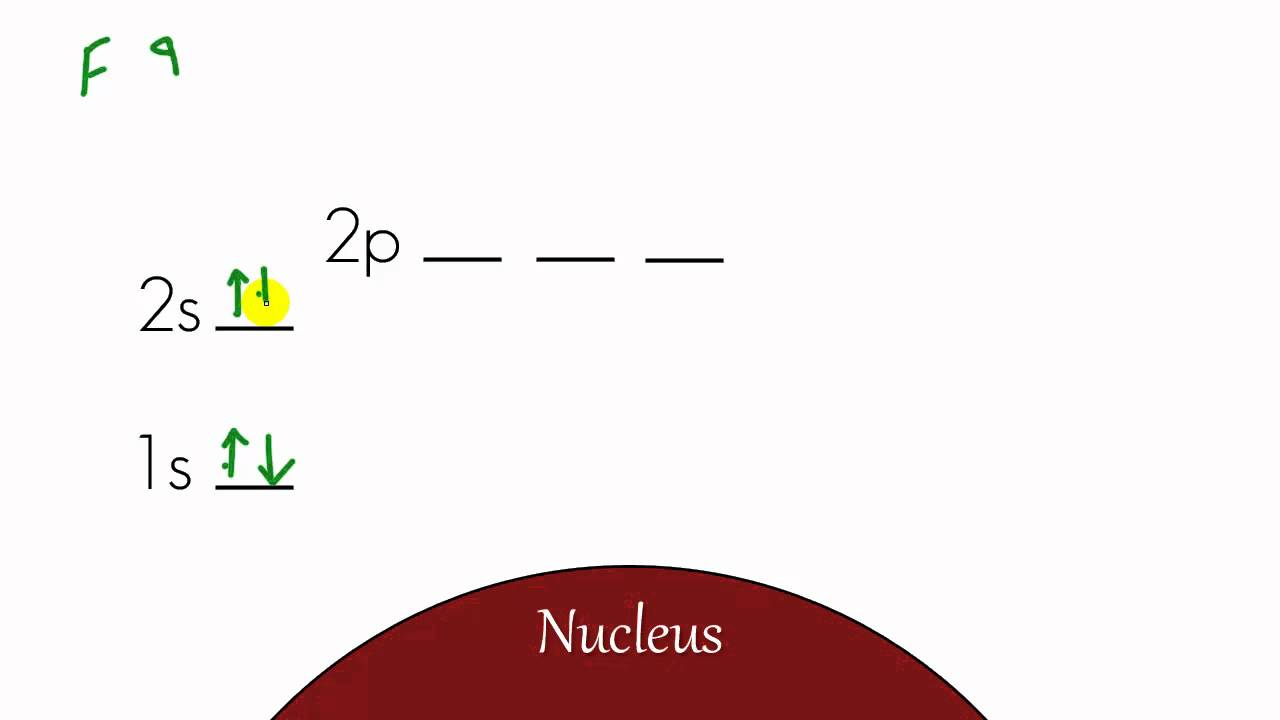
Chemistry Lesson - 12 - Energy Level Diagram and Electron Configuration

Which electron jump in a hydrogen atom absorbs the photon of highest

Electron Energy Levels of Atoms | Image License | Carlson Stock Art